Speed of enrollment indicates patients are seeking minimally invasive solutions to treat carpal tunnel syndrome
(Eagan, MN) — Feb. 22, 2023 — Sonex Health, a leader in the ultrasound-guided treatment of common orthopedic syndromes affecting the extremities, including entrapment neuropathies and tendinopathies, today announced the completion of patient enrollment in its clinical trial comparing ultrasound guided carpal tunnel release (CTR) versus traditional surgical mini-open CTR release (TUTOR). The TUTOR trial is the first multicenter randomized controlled trial in the United States to compare the efficacy and safety of traditional mini-open CTR (mOCTR) as compared to CTR using the FDA-cleared UltraGuideCTR™ with real-time ultrasound guidance.
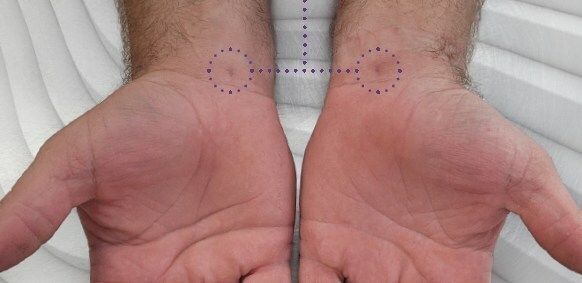
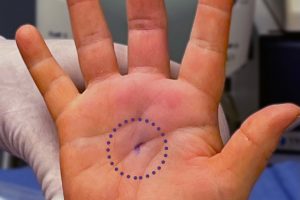
Carpal tunnel release with Sonex Health’s UltraGuideCTR and real-time ultrasound guidance allows for a small incision, typically closed without sutures. Carpal tunnel release with Sonex Health’s UltraGuideCTR and real-time ultrasound guidance allows for a small incision, typically closed without sutures. The TUTOR trial enrolled 122 patients who were randomized to receive one of the two CTR procedures at 12 clinical investigational sites. As part of this trial, patients will be followed for one year post procedure to gather data about the efficacy of the procedure, speed of recovery, return to activities, return to work and other factors.
“We are very grateful to all of the patients who chose to participate in the TUTOR trial, and to the investigators and their staff who committed their time and expertise to advance patient care for the treatment of carpal tunnel syndrome,” said Bob Paulson, CEO of Sonex Health. “Completing enrollment in this trial is a major milestone, and we thank the investigators and their teams for fully enrolling the TUTOR trial in just 82 days.”
UltraGuideCTR is a single-use, hand-held device developed by the physician co-founders of Sonex Health – Dr. Darryl Barnes and Dr. Jay Smith – both of whom previously practiced at the Mayo Clinic in Rochester, Minn., and founded The Institute of Advanced Ultrasound Guided Procedures, which focuses on product innovation, clinical research, and educating physicians on the application of musculoskeletal ultrasound. The UltraGuideCTR device for the treatment of carpal tunnel syndrome received FDA clearance in 2019.
“The speed at which patients enrolled in this trial demonstrates that people suffering from carpal tunnel syndrome are interested in minimally invasive options to alleviate their symptoms and return to normal activities as soon as possible,” said Dr. Kyle R. Eberlin, associate professor of surgery at Harvard Medical School, a plastic and reconstructive surgeon at Massachusetts General Hospital and the study’s principal investigator. “The TUTOR study has provided investigators with the opportunity to assess and compare two options to treat carpal tunnel syndrome and fully understand the efficacy and impact on each patient’s quality of life. It is an honor to be making a difference in our patients’ lives by expanding options to treat carpal tunnel syndrome.”
TUTOR investigators and sites include:
The study’s principal investigator: Kyle R. Eberlin, MD – Massachusetts General Hospital, Boston, Mass.
Christopher J. Dy, MD, MPH, FACS – Washington University Physicians, St. Louis, Mo.
Mark D. Fischer, MD – Twin Cities Orthopedics, Minneapolis, Minn.
James L. Gluck, MD – Kansas Orthopaedic Center, Wichita, Kan.
F. Thomas D. Kaplan, MD, FAAOS – Indiana Hand to Shoulder, Indianapolis, Ind.
Thomas J. McDonald, MD – Sierra Orthopedic Institute, Sonora, Calif.
Alexander Palmer, DO – Sano Orthopedics, Kansas City, Mo.
Marc E. Walker, MD – University of Mississippi Medical Center, Jackson, Miss.
James F. Watt, DO – Orthopaedic Associates, Destin, Fla.
Thomas P. Berkbigler, DO – Midwest Orthopedic Group, Farmington, Mo.
Paul E. Perry, MD – Tri-State Orthopaedics, Evansville, Ind.
Benjamin P. Amis, MD – ATX Orthopedics, Austin, Texas
Sonex Health is dedicated to improving the way in which patients receive care for carpal tunnel syndrome and is invested in continuing to prove the efficacy and improved quality of life that UltraGuideCTR and real-time ultrasound guidance provides patients through ongoing clinical data. For more information, please visit www.sonexhealth.com.
ABOUT SONEX HEALTH
Founded in 2014, the mission of Sonex Health is to be a world leader in ultrasound guided surgery by delivering physicians innovative therapies that reduce invasiveness, improve safety, and reduce the cost of care. With a strong focus on common orthopedic syndromes affecting the extremities, including entrapment neuropathies and tendinopathies, Sonex Health’s first proprietary technology — developed by Dr. Darryl E. Barnes and Dr. Jay Smith at the world-renowned Mayo Clinic — is UltraGuideCTR (formerly referred to as SX-One Micro-Knife), which may be utilized with or without ultrasound guidance to perform carpal tunnel release. Sonex Health’s second proprietary technology is UltraGuideTFR™, for the treatment of trigger finger, also known as stenosing tenosynovitis. For information about Sonex Health, UltraGuideCTR, and UltraGuideTFR visit www.sonexhealth.com.
ABOUT THE INSTITUTE OF ADVANCED ULTRASOUND GUIDED PROCEDURES
Founded in 2018 to support the Sonex Health mission and clinical excellence, The Institute of Advanced Ultrasound Guided Procedures is focused on innovation supported by robust clinical research and world-class professional education and training that transforms the treatment experience for patients, providers, and payers. For more information about the Institute visit www.sonexhealth.com/educational-institute.